News
-
Apr 29, 2025
C3i Meets the Next Generation of Scientists at INRS
Read MoreAn inspiring presentation on the role of CDMOs in Québec’s biotechnology ecosystem On Monday, April 28, C3i had the pleasure of presenting its mission and expertise at INRS – Institut national de la recherche scientifique, specifically at the Armand-Frappier Santé Biotechnologie Research Centre, located in Laval. This cutting-edge center is renowned for contributing to biomedical […]
-
Apr 09, 2025
C3i Attends the 2025 Cancer Immunotherapy Summit in Toronto
Read MoreA key moment of networking and collaboration to advance research C3i was pleased to participate in the Summit for Cancer Immunotherapy, held from April 6 to 8, 2025, in Toronto, a major event organized by BioCanRx. This annual summit brings together researchers, clinicians, patient partners, and industry leaders to discuss progress in cancer immunotherapy and […]
-
Mar 24, 2025
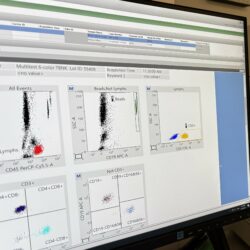
A Deep Dive into Flow Cytometry
Read MorePrecision-Driven Flow Cytometry: Advanced Technologies Ensuring Reliable Data for Complex Therapies. All flow cytometry assays performed in C3i are qualified and/or validated. Depending on the assays, three instruments are currently used: FACS Lyric, FACS Canto, or Accellix. During the qualification/validation, accuracy is verified using a known control (where reference values have already been established). Reproducibility […]
-
Mar 10, 2025
C3i announces the promotion of Julie Artigalas to Senior Manager – Client Accounts
Read MoreC3i is pleased to announce the promotion of Julie Artigalas to the position of Senior Manager – Client Accounts. This promotion marks a key milestone in our ongoing commitment to delivering strategic support and high-quality service to our partners, and reflects the significant impact Julie has had on the management of our clinical projects. Holding […]
-
Feb 19, 2025
C3i Center receives approval from the EMA to manufacture cell therapy products for the European market.
Read MoreC3i announces that it has obtained regulatory approval from the EMA (European Medicine Agency) to produce cell therapy treatments for the European market. The company received a certificate of Good Manufacturing Practice (GMP) compliance following an inspection of their Montreal facility by the European Medicines Agency. Therefore, C3i obtains a certificate of GMP (Good Manufacturing […]
-
Jan 28, 2025
C3i was proud to participate in Advanced Therapies Week, which took place in Dallas from January 20 to 23, 2025.
Read MoreAs a key event in the biotech industry, ATW 2025 gathered 2,500 attendees, 100 investors, 600 biotech companies, and 180 exhibitors at the Kay Bailey Hutchinson Convention Center. With six content tracks and 250 expert speakers, the event fostered innovation, regulatory discussions, and strategic collaborations to advance the future of cell and gene therapies. This […]
-
Jan 22, 2025
C3i Announces the Promotion of Jamie Sharp to Associate Director of Business Development
Read MoreC3i is pleased to announce Jamie Sharp’s promotion to Associate Director of Business Development. This milestone marks a key step in our growth strategy and reflects Jamie’s significant impact on our company’s development. Jamie Sharp is a biotechnology and cell and gene therapy specialist for CDMOs. Holding a Ph.D. with a background in chemical engineering […]
-
Oct 21, 2024
C3i appoints Yvan Côté as Chief Executive Officer, Paving the way for Strategic Growth and Innovation.
Read MoreThis fall has brought a new dynamic to C3i with the appointment of our new CEO, Yvan Côté. His expertise and leadership reinforce our commitment to elevating C3i to new heights. With a background in biotechnology and cell biology, combined with his strong business development expertise, Yvan has played a pivotal role in the significant […]
-
Jun 20, 2024
C3i Center Inc is the First CDMO in Canada to Receive Approval for a Drug Establishment License to Commercially Produce Cell Therapy Drug Products.
Read MoreWe are very proud to announce that we have received regulatory approval, in the form of a Drug Establishment License (DEL), to commercially produce cell therapies. This makes us the first CDMO in Canada to achieve this milestone. The approval follows an inspection by the cell and gene therapy experts from Health Canada. The DEL […]